DYSURE SUSPENTION

DIHYDROARTEMISININ PIPERAQUINE FOR ORAL SUSPENSION
DESCRIPTION
DYSURE SUSPENSIONS is a treatement of malaria. It is the fixed combination of Dihydroartemisinin & Piperaquine
COMPOSITION
Each 80 ml of reconstituted suspension contains :
| Dihydroartemisinin | 80 mg |
| Piperaquine Phosphate | 640 mg |
| Excipients | q.s |
| Colour | Sunset Yellow |
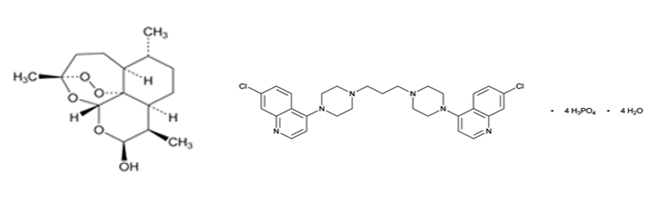
CHEMICAL STRUCTURE
PHARMACODYNAMICS
DHA is able to reach high concentrations within the parasitized erythrocytes. Its endoperoxide bridge is thought to be essential for its antimalarial activity, causing free-radical damage to parasite membrane systems including:
- Inhibition of falciparum sarcoplasmic-endoplasmic reticulum calcium ATPase,
- Interference with mitochondrial electron transport
- Interference with parasite transport proteins
- Disruption of parasite mitochondrial function
The exact mechanism of action of piperaquine is unknown, but it likely mirrors that of chloroquine, a close structural analogue. Chloroquine binds to toxic haeme (derived from the patient’s haemoglobin) within the malaria parasite, preventing its detoxification via a polymerisation step.
Piperaquine is a bisquinoline, and this class has shown good antimalarial activity against chloroquine- resistantPlasmodium strains in vitro. The bulky bisquinolone structure may be important for activity against chloroquine- resistant strains, and may act through the following mechanisms:
Piperaquine is a bisquinoline, and this class has shown good antimalarial activity against chloroquine- resistantPlasmodium strains in vitro. The bulky bisquinolone structure may be important for activity against chloroquine- resistant strains, and may act through the following mechanisms:
- Inhibition of the transporters that efflux chloroquine from the parasite food vacuole
- Inhibition of haem-digestion pathway in the parasite food vacuole.
THERAPEUTIC INDICATION
This medicine is a compound antimalarial drug recommended for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax malaria, in particular, in the case of resistance to other antimalarials
DOSAGE & ADMINISTRATION
- For children and infants.
- Add proper water until the volume of bottle (80ml) to reconstitute oral suspension. Shake well before use. Recap the bottle immediately after use. The treatment duration is 2 days.
- Dosage should be adapted according to weight
CONTRA-INDICATION
Hypersensitivity to one of the Excipients, pregnancy, severe liver or renal impairment, hematopathy (e.g. leucopenia or thrombocytopenia).
DRUG INTERACTION
Dysure is contraindicated in patients already taking other medicinal products that are known to prolong the QTc interval due to the risk of a pharmacodynamic interaction leading to an additive effect on the QTc interval.
Drug-drug pharmacokinetic interaction studies with Dysure have not been performed. The assessment of the potential for drug-drug interactions to occur is based on in vitro studies.
Drug-drug pharmacokinetic interaction studies with Dysure have not been performed. The assessment of the potential for drug-drug interactions to occur is based on in vitro studies.
STABILITY : See expiry on the pack
STORAGE : Store in a cool dry dark place. Protect from moisture.
PRESENTATION : 1 x 80 ml. Bottle in a Mono Pack
STORAGE : Store in a cool dry dark place. Protect from moisture.
PRESENTATION : 1 x 80 ml. Bottle in a Mono Pack

