ARTESUNATE INJECTION
COMPOSITION:
Each RAVYMAL 30 mg box contain 1 vial of 30 mg artesunat e powder for solution for injection, 1 ampoule of 1 ml sodium bicarbonate 5% w/v for injection and 1 ampoule of 5 ml sodium chlorideO.9% w/v for injection
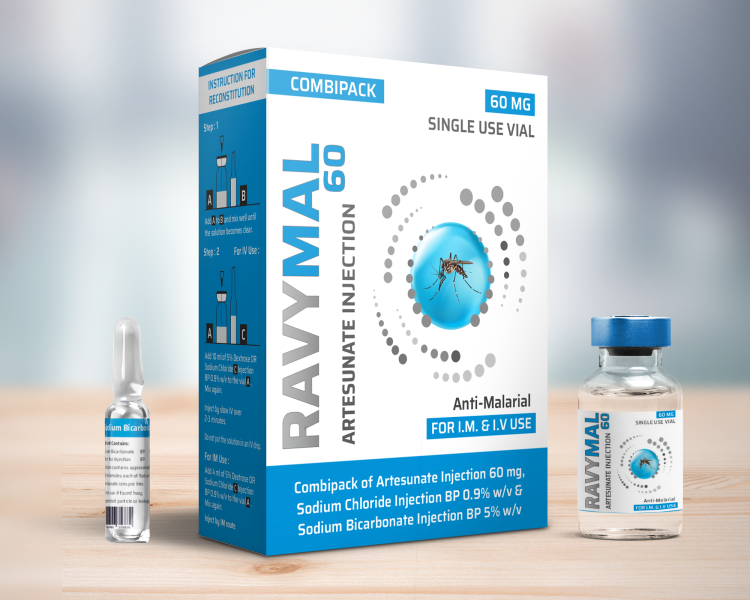
Each RAVYMAL 60 mg box contains 1 vial of 60 mg artesunate powder for solution for injection, 1 ampoule of 1 ml sodium bicarbonate 5% w/v for injection and 1 ampoule of 5 ml sodium chlorideO.9% w/v for injection.
Each RAVYMAL 120 mg box contains 1 vial of 120 mg artesunate powder for solution for injection, 1 ampoule of 2 ml sodium bicarbonate 5% w/v for injection and 1 ampoule of 10 ml sodium chiorideO.9% w/v for injection.
Each RAVYMAL 180 mg box contains 1 vial of 180 mg artesunate powder for solution for injection, 1 ampoule of 3 ml sodium bicarbonate 5% w/v for injection and 2 ampoule of 7.5 ml sodium Chloride 0.9% w/v for injection.
Effects on ability to drive and use of machines
There is no information on the effect of artesunate on the ability to drive or use machines. The patient’s clinical status should be considered when assessing ability to drive or operate machinery.
Undesirable Effects: Artesunate May Cause, Gast rointestinal Disturbances, Dizziness, Headache, Fever, Muscle And Joint Pain, Pruritas, Rarely Rash, Post Treatment Haemolytic Anaemia.
Overdose
Experience of acute overdose with artesunate is limited. A case of overdose has been documented in a 5-year-old child who was in advertently administered rectal artesunate at a dose of 88 mg/kg/day over 4 day, representing a dose more than 7-fold higher than the highest recommended artesunate dose. The overdose was Associated with pancytopenia, melena, seizures, multiorgan failure8 death. Treatment of overdose should consist of general supportive measures.
PHARMACEUTICAL PARTICULARS
List of excipients
Solvent: sodium bicarbonate
Diluents: sodium chloride
Incompatibilities In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products,
Shelf life
36 months
Special precautions for storage
Store below 30″C. Protect from light. Do not allow to freeze.
The reconstituted solution should be stored below 30″C and should be used within 1 hour
Keep out of reach of children.
PRESENTATION :
1) Combipack of 1 vial of 30mg Artesunate powder, 1 Ampouleof 1 ml Sod ium Bicarbonate solution g 1 ampoule of 5 ml Sodium chloride solution
2) Combipack of 1 vial of 60 mg Artesunate powder, 1 Ampoule of 1 ml Sodium Bicarbonate solution & 1 ampoule of 5 ml Sodium chloride solution
3) Combipack of 1 vial of 120mgArtesunatepowder, 1 Ampouleof2 ml Sodium Bicarbonate solution & 1 ampoule of 10ml Sodium Chloride solution

